Abstract
Background: InO is an anti-CD22 bispecific antibody conjugated with the cytotoxic agent calicheamicin, which despite its superior activity in patients (pts) with B-cell ALL poses the risk for hepatic VOD, especially in pts who proceed to stem cell transplantation (SCT) (Kantarjian et. al. Lancet Haem, 2017). Recommendations for reducing the risks of VOD in InO exposed pts have been developed, including fractionated weekly dose and overall dose reduction of InO as well as routine use of ursodiol prophylaxis (Kebriaei et.al. BMT 2018). Understanding disease and treatment factors that affect the risks of VOD in these pts needs to be evaluated further.
Aim: We aimed to assess factors that predict the subsequent development of VOD in adult pts with B-cell ALL treated with InO containing regimens.
Methods: From Jan 2010- Oct 2022, we analyzed pts with B-cell ALL treated on two clinical trials with InO containing regimen having prospectively collected data. We captured age, baseline liver function, InO dose exposure, SCT status and subsequent VOD incidences at any timepoint after InO exposure. VOD was diagnosed based on standard EBMT criteria for post SCT cases and a combination of clinical/radiological +/- liver histopathological diagnosis extrapolated from the EBMT criteria in non-SCT patients.
Results: 245 pts were reviewed who had InO exposure as part of 2 clinical trials during their B-cell ALL therapy. The median (med) age of the pts were 55 years (yrs) (18-88 yrs). A total of 172 pts (71%) received InO as part of their salvage therapy and the remaining 29% pts received InO as part of their frontline therapy; 81 pts (33%) received InO as monotherapy (+/- rituximab) and 164 pts (67%) received InO in combination with low-intensity chemotherapy (miniHCVD). Pts after 2015 received ursodiol as primary prophylaxis against VOD. The med InO exposure was 3.1 mg/m2 of body surface area (0.5- 9 mg/m2); med dose was 3.6 mg/m2 and 2.7 mg/m2 in the monotherapy and combination arms respectively. The med time to InO initiation from diagnosis was 8.3 mos (12.7 mos in monotherapy vs. 1.9 mos in combination arm). Overall, 199 pts (81%) obtained a response (CR/CRi/CRp) with InO based therapy. With a med follow up duration of 68 months (mos) for the entire cohort, 75 pts (31%) had undergone a SCT. The med time to SCT from last InO cycle was 1.7 mos (0.6.- 7.6 mos) and 17.3 mos from diagnosis. Nineteen pts (25%) received blinatumomab as part of the treatment protocol after InO and before proceeding to SCT.
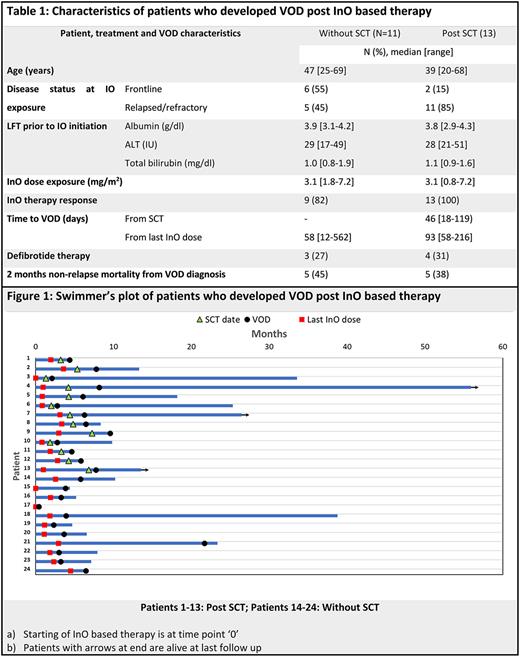
A total of 24 pts (9.7%) had a diagnosis of VOD during their follow up, 13 (54%) post-SCT and one pt (4%) post CART therapy (Table 1). The med time to the onset of VOD from SCT in the 13 transplanted pts was 46 days (18-119 days) and the med time to SCT from last InO dose was 43 days (vs. 53 days in those transplanted pts who did not have a VOD, p=0.9). Overall, the med time to VOD from last InO dose was 79 days (12-562 days). The med age of the pts who developed VOD was 40 yrs at the time of InO exposure and their med InO exposure was 3.1 mg/m2 (0.8-7.2gm/m2). Only one pt had acute onset VOD within 2 weeks of InO exposure and succumbed to it shortly after. Of the remaining 23 pts, 22 (96%) were responders [CR/CRi, (12/19 MRD-ve at response)] with the InO based regimen. On univariate analysis, including pt. age, serum albumin, total bilirubin and ALT before InO initiation, InO dose exposure, initial response to InO based therapy, and SCT, only SCT was significantly associated with higher odds of VOD (odds ratio= 3.5, 95% CI=1.5-8.2). On multivariate analysis, the effect of SCT on VOD became insignificant. Ten pts (42%) died within 60 days of diagnosis of VOD (one early death after InO initiation before response assessment, 5 pts after SCT while in remission, 1 pt after CART therapy in remission, 2 pt in remission without SCT/CART and 1 pt with persistent disease) (Fig.1). At the time of data cutoff only 3 pts (11%) are alive with resolution of their VOD (one pt had received defibrotide). Across the whole cohort, the med OS for pts who had VOD was 8.9 mos vs. 15.mos in those without VOD.
Conclusion: VOD remains a dreaded, but rare complication (<10%) of therapy in pts treated with InO based regimens and higher incidence post SCT. Fractionation of InO doses, avoiding concurrent azole antifungals and routine use of ursodiol prophylaxis during InO therapy and peri-SCT period might alleviate these risks. Further studies are ongoing to develop a prediction model for InO induced VOD.
Disclosures
Jabbour:AbbVie: Other: Advisory Role, Research Funding; Adaptive Biotechnologies: Other: Advisory Role, Research Funding; Amgen: Other: Advisory Role, Research Funding; Bristol Myers Squibb: Other: Advisory Role, Research Funding; Genentech: Other: Advisory Role, Research Funding; Pfizer: Other: Advisory Role, Research Funding; Spectrum: Research Funding; Takeda: Other: Advisory Role, Research Funding. Short:Takeda Oncology: Consultancy, Research Funding; AstraZeneca: Consultancy; Stemline Therapeutics: Research Funding; Amgen: Consultancy, Honoraria; Pfizer: Consultancy; Novartis: Consultancy; Astellas: Research Funding. Jain:Newave: Research Funding; TransThera Sciences: Research Funding; Beigene: Honoraria; Cellectis: Honoraria, Research Funding; TG Therapeutics: Honoraria; Dialectic Therapeutics: Research Funding; Novalgen: Research Funding; Loxo Oncology: Research Funding; Medisix: Research Funding; Takeda: Research Funding; Mingsight: Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Fate Therapeutics: Research Funding; Aprea Therapeutics: Research Funding; Pfizer: Research Funding; Incyte Corporation: Research Funding; Cellectis: Honoraria, Research Funding; BMS: Consultancy, Honoraria, Other: Travel Support, Research Funding; ADC Therapeutics: Research Funding; Servier Pharmaceuticals LLC: Research Funding; Precision Biosciences: Consultancy, Honoraria, Other: Travel Support, Research Funding; Pharmacyclics, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria, Other: Travel Support; Genentech, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding; AstraZeneca: Consultancy, Honoraria, Other: Travel Support, Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Other: Travel Support, Research Funding; AbbVie: Consultancy, Honoraria, Other: Travel Support, Research Funding; MEI Pharma: Honoraria; Ipsen: Honoraria; CareDx: Honoraria. Kebriaei:Kite: Consultancy; Amgen: Research Funding; Pfizer: Consultancy; Jazz: Consultancy; Ziopharm: Research Funding. Champlin:Actinium: Consultancy; General Oncology: Other: Data Safety Monitoring Board; Bluebird: Other: Data Safety Monitoring Board; Kadmon: Consultancy; Cell Source Inc.: Research Funding; Omeros: Consultancy; Johnson &Johnson: Consultancy. Shpall:Navan: Consultancy; axio: Consultancy; Takeda: Patents & Royalties; Affimed: Other: License agreement; NY blood center: Consultancy; adaptimmune: Consultancy; Bayer: Honoraria; Fibroblasts and FibroBiologics: Consultancy. Kantarjian:AbbVie: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Consultancy, Research Funding; ImmunoGen: Research Funding; Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Research Funding; KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; NOVA Research: Honoraria; Novartis: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Takeda: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal